PROFILE
Established in 1982, Genius is arguably the oldest health care biotechnology company in South Africa, if not the African continent, with a track record of investing in research and development work that led to successfully commercialising South Africa’s first biosimilar product, Repotin, a recombinant human hormone known as erythropoietin (EPO).
Genius focuses on health care applications and more specifically on the production of biopharmaceutical products. Biopharmaceutical products include such therapeutics as hormones, protein-based drugs and antibodies.
Genius is a research and development biopharmaceutical group of companies which includes the following operational business units:
- Immunotherapy dendritic cell vaccine technology (DCV)
- Erythropoietin production, registered as Repotin
- Granulocyte-colony stimulating factor (G-CSF) product development
- Integrated Bioworks (IBW) reagents
2017 OPERATIONAL PERFORMANCE
The divisions are engaged in product development which are at different stages and the progress is as follows:
- Regulatory preparation and process validation phase for the production of EPO, a biosimilar drug used to treat anaemia caused by kidney disease
- Developmental work on DCV for cancer immunotherapy and non-communicable diseases such as extreme drug-resistant tuberculosis
- Further developmental activities at the Ribotech facility for a second biosimilar drug called Recogen, a G-CSF used for the treatment of neutropenia
- Production of protein-based reagents used mainly in research laboratories and industrial applications.
VISION 2020 VISION – GENIUS’ CONTRIBUTION
The outlook for Genius and their value proposition is based on a long-term vision and value-add that is linked to defined milestones.
Unlike other typical investments in other sectors, Genius works to achieving milestones in their developmental stage, hence a five-year strategic plan to the year 2020 was implemented with clear deliverables and strategic actions for each strategic lever as well as detailed clear time frames.
- The cell culture division is currently manufacturing EPO and is undergoing regulatory preparation with “mock-runs”
- The DCV project is preparing for phase 1 human clinical trial in breast cancer patients and performing pre-clinical trials on X‑DR tuberculosis
- Further development work for the production of a second biotech drug for humans
PROSPECTS AND FUTURE OUTLOOK
The following prospects have been identified:
SHORT-TERM PROSPECTS:
- For Repotin production, processes, updates and regulatory framework are to be completed by 2018
- Secure funds to complete final filling solutions for recombinant products for both Repotin and Recogen
- Further research and development work in order to continue to production phase
- Begin the phase 1 human clinical trial in breast cancer patients and pre-clinical work on other diseases
- Integrated Bioworks to generate revenue from the sale of laboratory reagents and increase its product offering
- Based on the phase 1 trial results – accelerate the listing of Genius to obtain the necessary capital to complete phase 2 and 3 trials
MEDIUM- TO LONG-TERM:
- Optimise and increase the production output of Repotin so that it is able to meet market demand by 2020 and introduce additional formulation strengths to enter the oncology market
- Introduce an additional range of recombinant and biologically derived products through a licensing agreement with an international partner
- Partner with a South African multinational pharmaceutical company to secure a sales and marketing channel for local and international biological markets
- Plan and prepare to list Genius on a foreign stock exchange
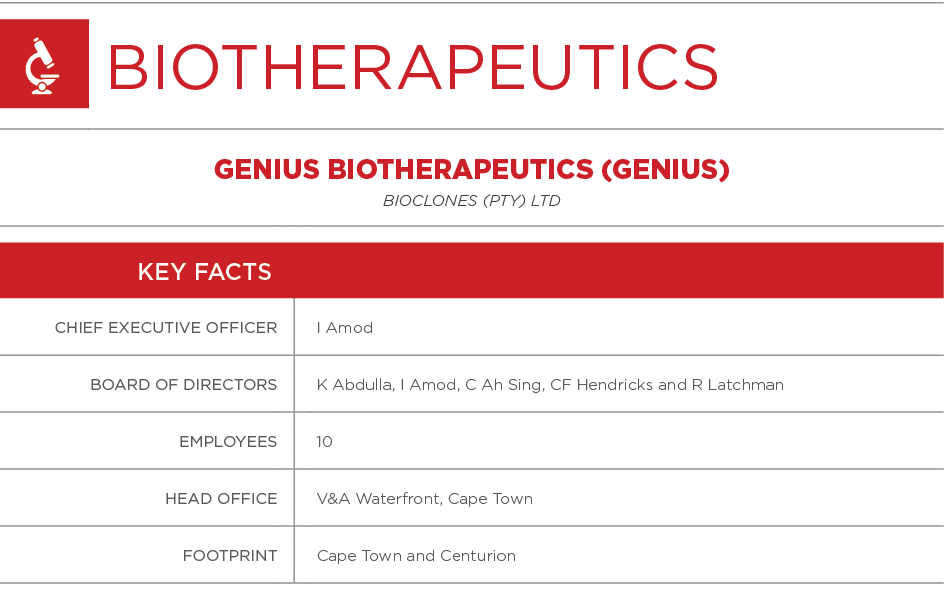
FOOTPRINT
The Genius Group has two state-of-the-art medium-sized biopharmaceutical laboratory facilities, one in Cape Town and the other in Centurion, Pretoria.
PRODUCTS/BRANDS

Detailed information about Genius’ business model, stakeholders, risks and governance is available on the website at www.aeei.co.za.

